Simple examples of redox reactions
Definition of redox reactions
Redox reactions define as electron transfer reactions. Thus when it proceeds one element loses electrons and other elements gain these electrons. The study of the standard electrode potentials helps in predicting the reactions possibly or not. Thus standard electrode potentials help to calculate the equilibrium constant of these reactions.
Examples of redox reactions
Let us take the simple example of the oxidation of ferric ions by ceric ions in the presence of dilute sulfuric acid. We can derive equilibrium constant in terms of thermodynamics free energy change of the reactions. If we apply the Nernst equation to the above equation
In the above case value of n=1, thus for the standard electrode potential of the half-cell reactions, we can easily calculate the equilibrium constant.
Electrochemical cell redox reaction
Electrochemical cell reactions provide the quantitative basis of the redox reaction. Thus this cell produces electrical energy by spontaneous oxidation-reduction reactions. Danial cell is an example of this type of cell. In this cell, electrons are transferred from the zinc atom to copper atom. Thus this cell consists two-electrode with the cell reactions
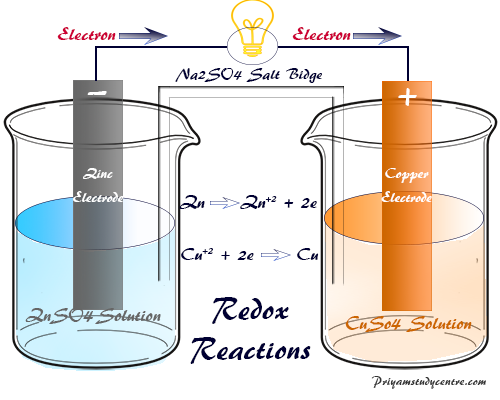
At the Zinc electrode
Zn(S) → Zn+2 +2e
At the copper electrode
Cu + 2(aq) + 2e → Cu(S)
Thus the electrode where occur loss of electrons called an anode but where occurs addition of electrons is called the anode.
The oxidizing agent in redox reactions
Ceric sulfate solution preparation
Ceric sulfate is a very good oxidizing agent used in redox reactions of the titration process.
Ce+4 during the reaction exists as an anionic complex in H 2SO 4 solutions. Thus the formal potential in sulfuric acid solution = 1.42 V.
Potassium permanganate solution
Potassium permanganate is a powerful oxidizing agent in the redox process. It is very useful for redox titration because no special indicator is needed for noted the endpoint of the redox reactions.
Originally published at https://www.priyamstudycentre.com on January 26, 2019.

