Zinc Element, Uses, Properties, Facts
Zinc in Periodic Table
Zinc (Zn), chemical element, the lustrous silvery metal of Group 12 or (IIB) of the periodic table, used in making alloys, corrosion-resistant coating, and dry cells. It is the last member of the 3d-series but no characteristics of the transition metals due to the presence of full fill d- orbital. The rise of shielding electrons along the 3d-series gradually makes the d-shell is a part of the inner core for the zinc atom and leaving only the s-pair for chemical bonding.
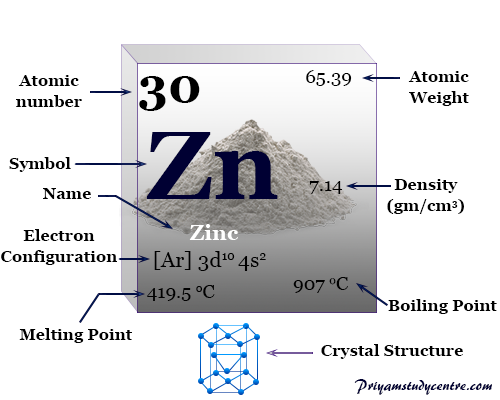
The hexagonal closed packed crystal lattice, zinc has the chemical symbol Zn, atomic number 30. The closed packed lattice with elongated distances between the layers makes the metal denser than those of the copper and silver. The physical and chemical properties like atomic weight, melting point, boiling point, density, and valence shell electronic configuration of zinc are given below the picture. The low melting and boiling point of the metal reflects the weak participation of the outer ns electron for metallic bonding.
History, Occurrence, and Isolation
The making of brass from copper and zinc ores was known from ancient times in Palestine, Greece, Rome, India, and China. The extraction of the metal at this time was difficult because the metal passes to the vapor state at the required reduction temperature (1000ºC). The name zinc may be related to the German word Zinke meaning spike or tooth. It also originates from the Latin word leucoma or white deposit.
Zinc is slightly more abundant (76 ppm) in the earth’s crust than copper (68 ppm). Zinc blended (ZnS) and calamine (ZnCO 3) are the most industrial important ores of the metal. The other minor important ores are franklinite (ZnO, Fe 2O 3) and willemite (Zn 2SiO 4). Canada, Russian countries, Australia, chain, Peru, and United States are the major producing countries of the metal.
Unlike iron, the reduction of zinc oxide by carbon is not effective due to its boiling point (907°C). In the electrolytic method, the ore is roasted at a lower temperature (650ºC) to form ZnSO 4. The roasted mass is extracted with dilute sulfuric acid and the extract treated with milk of lime to precipice iron, aluminum, and silica. Copper and cadmium are next precipitated by Zn-dust. The solution of ZnSO 4 electrolyzed by the aluminum anode and zinc cathode under high current density for the production of the pure metal.
Presently the metal is extracted by a specially designed blast furnace. The ore roasted to the oxide by coke which volatilizes with the hot blast. The gas is suddenly chilled by pouring molten lead on it. Therefore, the reoxidation of zinc during cooling becomes negligible and collected the liquid Zn on the bottom of the chamber. It is 99 percent pure and it further purified by vacuum distillation.
Chemical Properties of Zinc
The lustrous silvery metal, zinc belongs to 3d-block but they do not form any compound in which the d-shell is partially occupied. Due to poor shielding, the d-electrons pulls into the inner core. The ionization energy of the metal also explains the fact. The third ionization energy of zinc is considerably higher than that of the first or second one indicating stronger binding for the d-electrons. Therefore, the most stable and important oxidation state of Zn is +2.
Zinc shows certain similarities with the main group or s-block elements due to the presence of ns 2outer electronic configuration. However, the element resembles the transition metal because it forms several complex compounds with a variety of ligands like ammonia, amines, halides, and cyanides. But the complexes with other strong pi-acceptor ligands like carbonyl, nitrosyl, and olefins are not known. The metal readily reacts with oxygen, sulfur, phosphorus, and halogens to form a variety of simple chemical compounds.
Chemical Compounds
The large difference between the 1st and 2nd ionization energies of Zn, Cd, Hg suggests the formation of M+ion might be possible. In practice, the univalent state appears and important for mercury in the form of Hg 2+2. While the +2 state is favorable for zinc and cadmium atoms due to higher hydration energy or bond energy for +2 ion. Among group 12 elements of the periodic table, only mercury forms a limited number of compounds in the +1 state but other elements like zinc and cadmium from the chemical compounds in the +2 state.
The oxides, sulfides, and halides of Zn(II) are the most important binary compounds of the metal. It also forms unstable hydrides, nitrides, and carbides (acetylides). ZnH 2 has been isolated as a colorless solid by reduction of ZnBr 2 or ZnI 2 with lithium hydride or sodium hydride in tetrahydrofuran. The normal oxides (ZnO) and peroxide (ZnO 2) formed by heating the metals or metal sulfide in the air.
The sulfide of zinc is very familiar to us in the routine group analysis of the cation and made by direct reaction of aqueous Zn(II) with hydrogen sulfide. It forms two crystalline solid forms like zinc blende or wurtzite and transforms each other at 1020°C… more about see the original post
Originally published at https://www.priyamstudycentre.com on January 17, 2021.
